| Anmol Chemicals is the pioneer manufacturers of Heavy Bismuth Subnitrate, Pharmaceutical Excipients Chemicals in India. We offer Halal and Kosher Heavy Bismuth Subnitrate made in an ISO9001, ISO22000 (FSSC22000) cGMP & GLP certified facility. Our group has several manufacturing facilities spread across the world, supported by toll manufacturers and representatives in UAE, Europe, Africa, USA, China and has several associated manufacturing facilities spread across India. All the Information on Physics, Chemistry, Applications, Uses and Technology on Manufacture of Heavy Bismuth Subnitrate is in these pages. |
| The units have one or more of the certifications like FDA GMP, ISO 9001, ISO 22000, HACCP, REACH, Kosher & Halal |




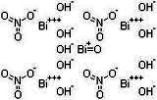
Heavy Bismuth Subnitrate BP USP Pure Grade Manufacturers
Heavy Bismuth Subnitrate CAS Number 1304-85-4, EINECS EC Number 215-136-8, HS Code ---**; Molecular Weight 1461.99, Molecular Formula: Bi5O(OH)9(NO3)4
SDS MSDS of Heavy Bismuth Subnitrate Manufacturers
Specifications of Bismuth Citrate Manufacturers
Specifications of Bismuth Subcarbonate Manufacturers
Specifications of Bismuth Subgallate Manufacturers
Specifications of Bismuth Subsalylate Manufacturers
Specifications of Heavy Bismuth Subnitrate Manufacturers. You are here.
We offer Heavy Bismuth Subnitrate of Commercial Pure and BP USP Grade.
Heavy Bismuth Subnitrate BP
Ph Eur
4[BiNO3(OH)2],BiO(OH) -- 1462 -- 1304-85-4
DEFINITION
Content: 71.0 per cent to 74.0 per cent of Bi (Ar 209.0) (dried substance).
CHARACTERS
Appearance: White or almost white powder.
Solubility: Practically insoluble in water and in ethanol (96 per cent). It dissolves in mineral acids with decomposition.
IDENTIFICATION
A. Dilute 1 ml of solution S1 (see Tests) to 5 ml with water and add 0.3 ml of potassium iodide solution. A black precipitate is formed which dissolves into an orange solution with the addition of 2 ml of potassium iodide solution.
B. It gives reaction (b) of bismuth.
C. It gives the reaction of nitrates.
D. pH: maximum 2.0 for solution S2 (see Tests).
TESTS
Solution S1: Shake 5.0 g by gently heating in 10 ml of water and add 20 ml of nitric acid. Heat until dissolution, cool and dilute to 100 ml with water.
Solution S2: Place 1.00 g in a 20 ml volumetric flask and add 2.0 ml of lead-free nitric acid. Allow acid attack to take place without heating and if necessary warm slightly at the end to completely dissolve the test sample. Add 10 ml of water, shake and add, in small fractions, 4.5 ml of lead-free ammonia; shake and allow to cool. Dilute to 20.0 ml with water, shake again is required to change the colour of the indicator to pink.
Chlorides: Maximum 200 ppm.
Copper: Maximum 50.0 ppm.
Lead: Maximum 20.0 ppm.
Silver: Maximum 25.0 ppm.
Substances not precipitated by ammonia: Maximum 1.0 per cent.
To 20 ml of solution S1, add concentrated ammonia until an alkaline reaction is produced and filter. Wash the residue with water and evaporate the combined filtrate and washings to dryness on a water-bath. To the residue, add 0.3 ml of dilute sulphuric acid R and ignite.
The residue weighs a maximum of 10 mg.
Loss on drying: Maximum 3.0 per cent, determined on 1.000 g by drying in an oven at 105C.
ASSAY: Dissolve with heating 0.250 g in 10 ml of a mixture of 2 volumes of perchloric acid and 5 volumes of water. To the hot solution, add 200 ml of water and 50 mg of xylenol orange triturate. Titrate with 0.1 M sodium edetate until a yellow colour is obtained.
1 ml of 0.1 M sodium edetate is equivalent to 20.90 mg of Bi.
Bismuth Subnitrate USP
Bi5O(OH)9(NO3)4 1461.99
Bismuth hydroxide nitrate oxide Bi5O(OH)9(NO3)4 [1304-85-4].
Bismuth Subnitrate is a basic salt that contains the equivalent of not less than 79.0 percent of bismuth trioxide (Bi2O3), calculated on the dried basis.
Packaging and storage: Preserve in well-closed containers.
Identification: It responds to the tests for Bismuth and for Nitrate.
Loss on drying: Dry it at 105 for 2 hours: it loses not more than 3.0% of its weight.
Carbonate: Add 3 g to 3 mL of warm nitric acid: no effervescence occurs. Pour the solution into 100 mL of water: a white precipitate forms. Filter, evaporate the filtrate on a steam bath to 30 mL, again filter the liquid, divide the latter filtrate into portions of 5 mL each, and use these several portions in the tests for Chloride, Sulfate, Copper, Lead, and Silver.
Chloride: A 10-mL portion of the test liquid retained in the test for Carbonate shows no more chloride than corresponds to 0.50 mL of 0.020 N hydrochloric acid (0.035%).
Sulfate: To a 5-mL portion of the test liquid retained in the test for Carbonate add 5 drops of barium nitrate: no turbidity is produced immediately.
Limit of ammonium salts: Boil about 100 mg with 5 mL of 1 N sodium hydroxide: the vapor does not turn moistened red litmus paper blue.
Arsenic, Method: Mix 375 mg with 5 mL of water, cautiously add 2 mL of sulfuric acid, and heat the mixture until fumes of sulfur trioxide are copiously evolved. Cool, cautiously add 10 mL of water, and again evaporate to strong fuming, repeating, if necessary, to remove any trace of nitric acid. The limit is 8 ppm.
Copper: To a 5-mL portion of the test liquid retained in the test for Carbonate add a slight excess of 6 N ammonium hydroxide: the liquid does not exhibit a bluish color.
Lead: Mix a 5-mL portion of the test liquid retained in the test for Carbonate with an equal volume of 2 N sulfuric acid: the liquid does not become cloudy.
Silver: To a 5-mL portion of the test liquid retained in the test for Carbonate add hydrochloric acid, dropwise: no precipitate is formed that is insoluble in a slight excess of hydrochloric acid, but that is soluble in 6 N ammonium hydroxide.
Limit of alkalies and alkaline earths: Boil 1.0 g with 20 mL of a mixture of equal volumes of 6 N acetic acid and water, cool, and filter. Add 2 mL of 3 N hydrochloric acid, precipitate the bismuth by the addition of hydrogen sulfide, boil the mixture, and filter it. Add 5 drops of sulfuric acid to the filtrate, evaporate to dryness, and ignite to constant weight: the weight of the residue does not exceed 5 mg (0.5%).
Assay: Transfer about 400 mg of Bismuth Subnitrate, accurately weighed, to a 250-mL beaker. Add 5 mL of water, then add 2 mL of nitric acid, and warm, if necessary, to effect solution. Dilute with water to 100 mL, add 0.3 mL of xylenol orange and titrate with 0.05 M edetate disodium VS to a yellow endpoint. Each mL of 0.05 M edetate disodium is equivalent to 11.65 mg of Bi2O3.
Manufacturers
Anmol Chemicals
S-8, SARIFA MANSION, 2ND FLANK ROAD, CHINCHBUNDER, MUMBAI 400009, INDIA
TEL: (OFFICE) 91-22-23770100, 23726950, 23774610, 23723564. FAX: 91-22-23728264
e-mail: anmolc@mtnl.net.in

Exports to USA, Canada, UAE, Dubai, South Africa, Tanzania, Kenya, Nigeria, Egypt, Uganda, Turkey, Mexico, Brazil, Chile, Argentina, Europe Netherlands, Italy, Spain, Germany, Portugal, France, Malaysia, Indonesia, Thailand, Vietnam, Korea, Japan, etc.
Copyright and Usual Disclaimer is Applicable. 12 January, 2022
Barter
They who love thee on this earth, keep calling on thee and chanting thy beads
Lest thou forgetest.
They assign the credit of their hard work to your blessings
They keep you amused.
They come to thy temple with baskets of fruits, as if you were a glutton
They deny the same to their children.
They offer you milk for a bath and burn the ghee
They hardly understand the meaning of it.
They bring gold and diamonds. They come with beating of drums
They love to advertise their offerings.
They offer you a small bribe as advance for booty, called lottery
They love to dream.
I do not bow down at your door
I do not bargain for booty
Your promise of the heaven does not lure me
Your hell-fire does not scare me
I do not even know the proper method of prayer
I do not offer any thing to you
Ages have gone by and I have not seen you my lord
Yet my love for you keeps waiting for you